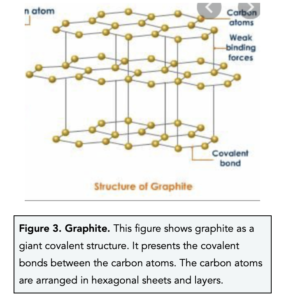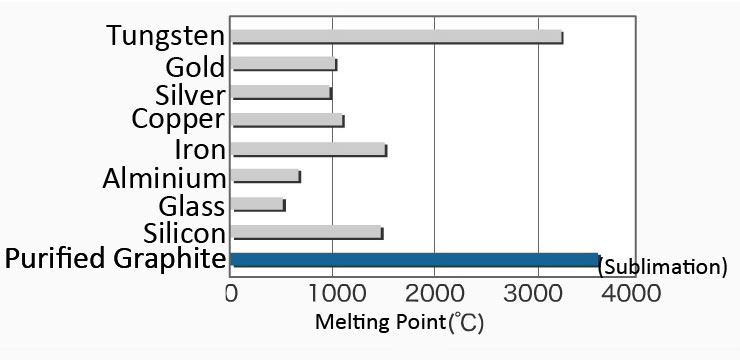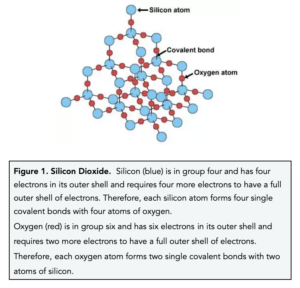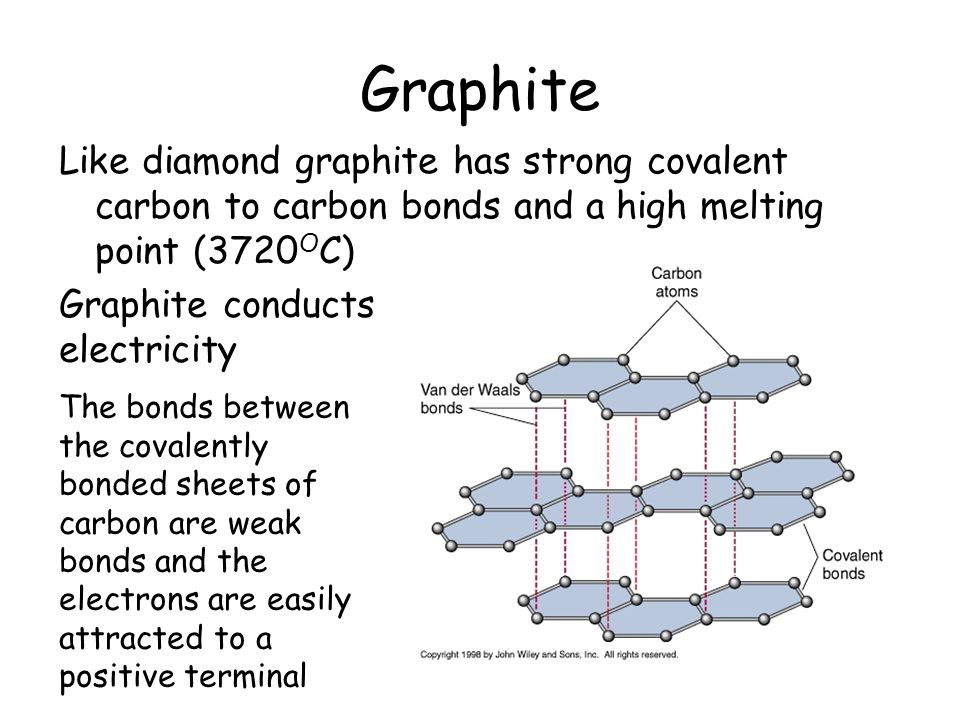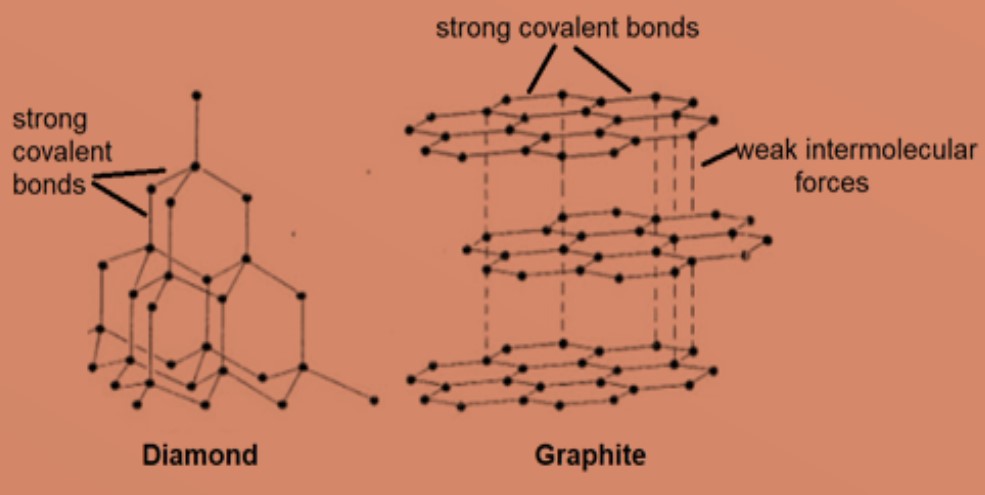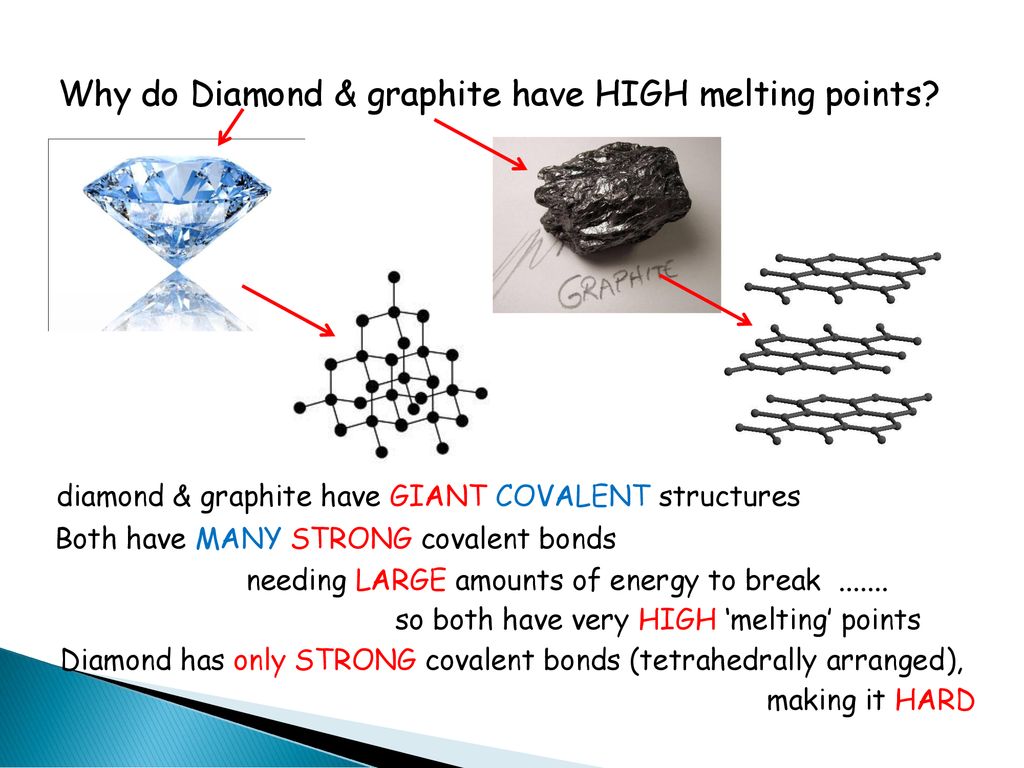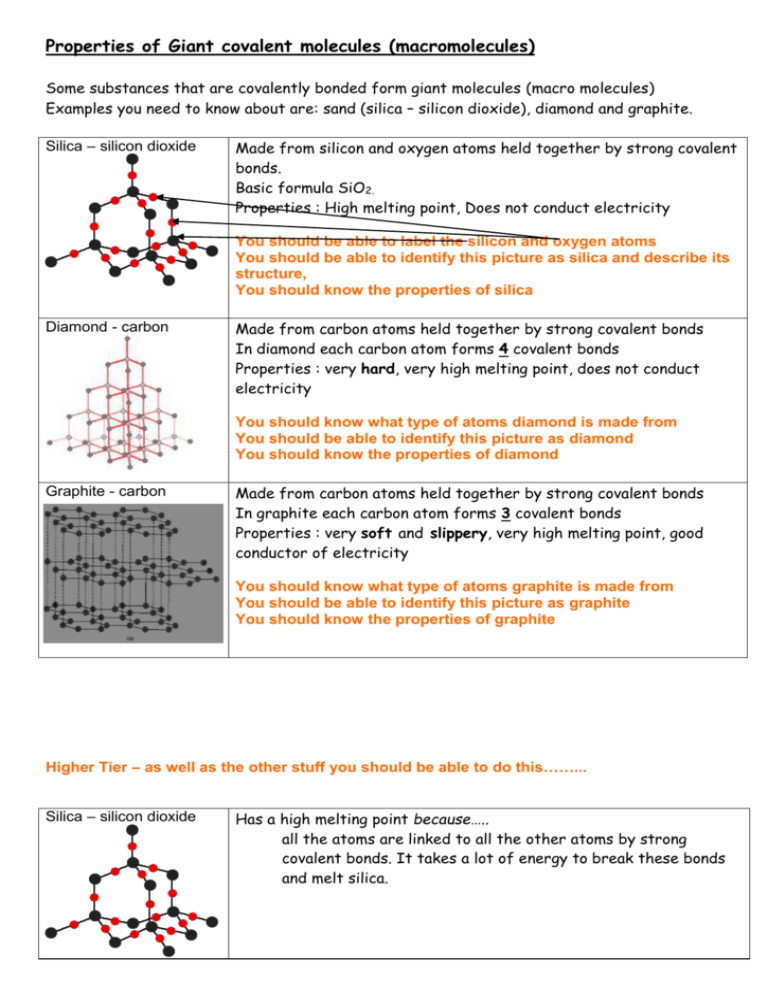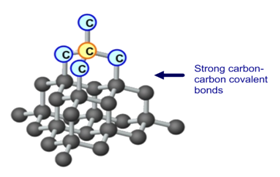
1:49 explain why substances with giant covalent structures are solids with high melting and boiling points - TutorMyself Chemistry

Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect