Selective preparation of bio-based high value chemical of p-tolylaldehyde with Cr(OH)3@Fe3O4 catalyst | SpringerLink

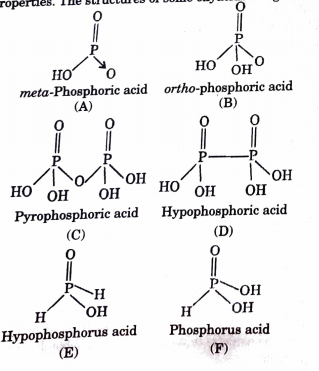
Metaphosphoric acid, 39-43%, bal. NaPO{3} (Stabilizer), Thermo Scientific Chemicals, Quantity: 100 g | Fisher Scientific

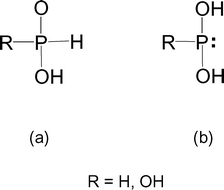
Phosphorous acid, H_3PO_3, has the structure (HO)_2PHO, in which one H atom is bonded to the P atom, and two H atoms are bonded to O atoms. For each bond to an

Why is phosphorous acid H3PO3 and not P(OH)3 - which should be more accurate as per the molecule structure? - Quora

Highly Efficient Low-Concentration Phosphate Removal from Effluents by Recoverable La(OH)3/Foamed Nickel Adsorbent | ACS Omega
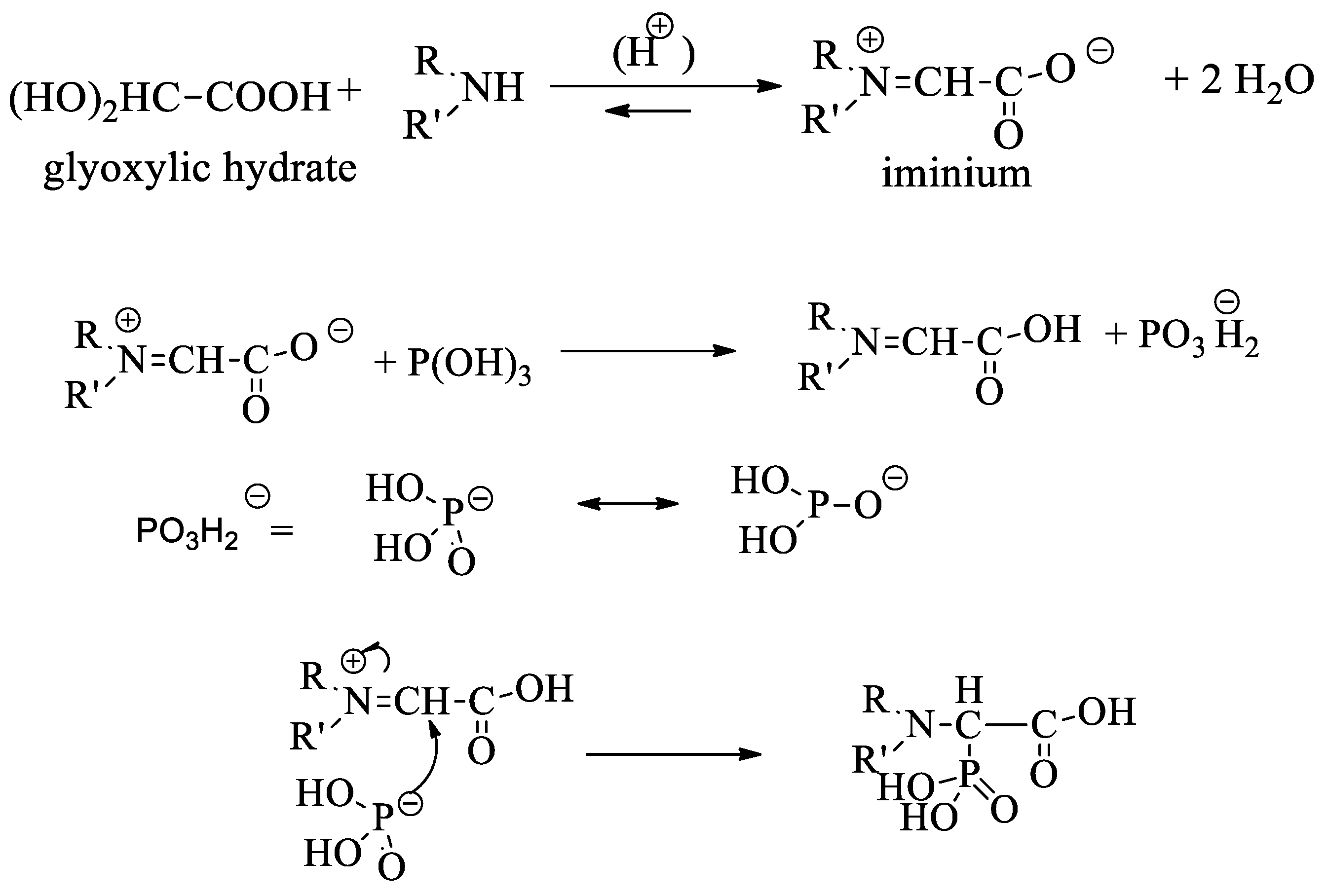
Organics | Free Full-Text | MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids
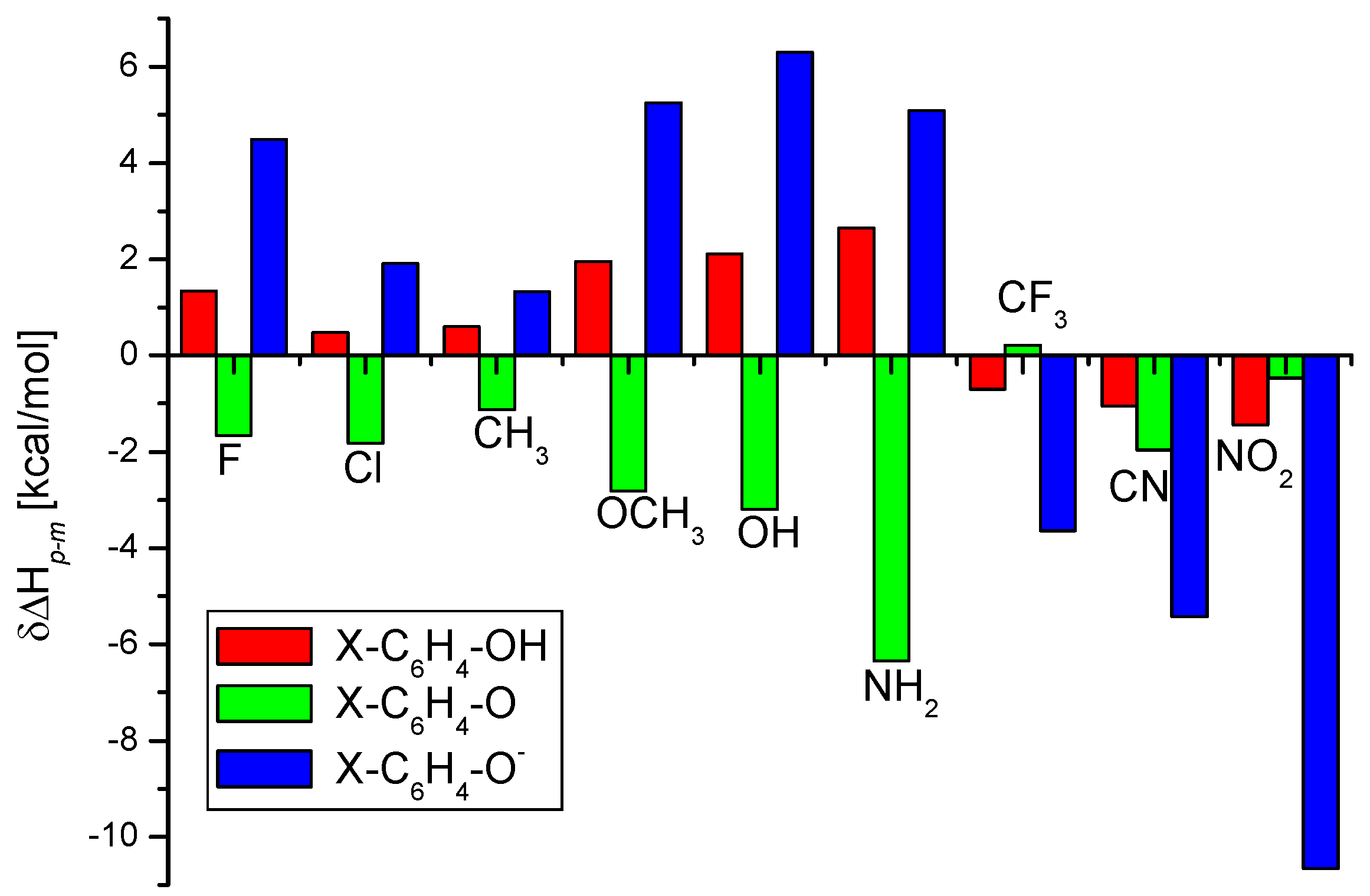
IJMS | Free Full-Text | The O-H Bond Dissociation Energies of Substituted Phenols and Proton Affinities of Substituted Phenoxide Ions: A DFT Study





![Q54E Phosphoric acid (H3PO4)is a t... [FREE SOLUTION] | StudySmarter Q54E Phosphoric acid (H3PO4)is a t... [FREE SOLUTION] | StudySmarter](https://studysmarter-mediafiles.s3.amazonaws.com/media/textbook-exercise-images/image_9jCIKPD.png?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIA4OLDUDE42UZHAIET%2F20230529%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20230529T000033Z&X-Amz-Expires=90000&X-Amz-SignedHeaders=host&X-Amz-Signature=53d3da6dfdf40bf065a0127fe3908055de6e981deda0a81acf97cb4f46b88b32)
![Solving For pH, pOH, [H+], [OH-] - Acids & Bases Solving For pH, pOH, [H+], [OH-] - Acids & Bases](http://youarebasic.weebly.com/uploads/5/0/1/4/50143245/5802360_orig.png)

![Calculating pH, pOH, [H+] and [OH-] of Common Substances | TPT Calculating pH, pOH, [H+] and [OH-] of Common Substances | TPT](https://ecdn.teacherspayteachers.com/thumbitem/Calculating-pH-pOH-H-and-OH-of-Common-Substances-3600412-1657170541/original-3600412-3.jpg)