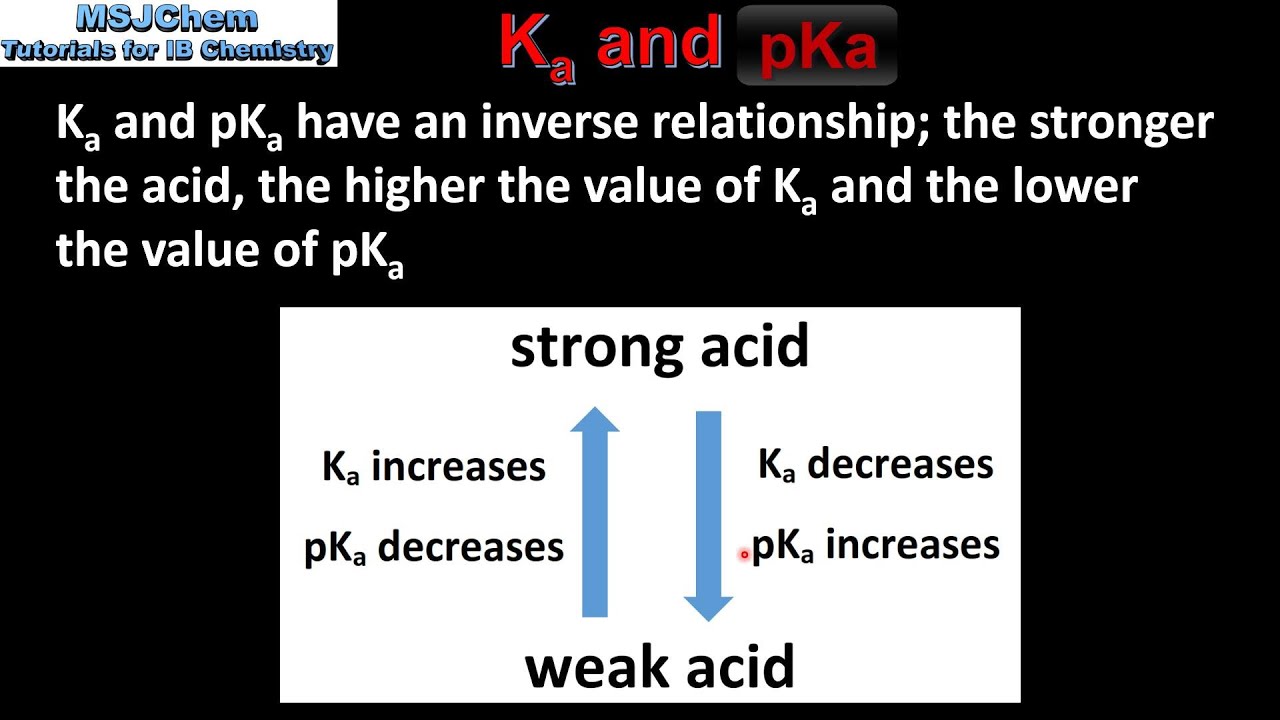
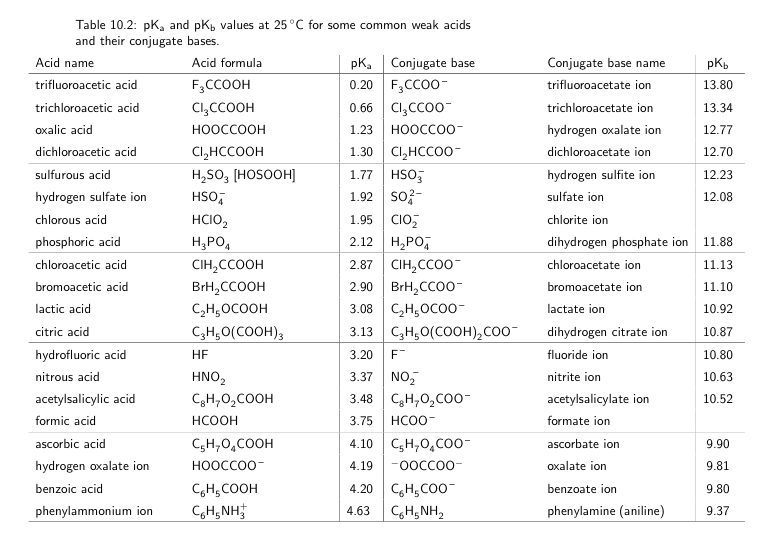
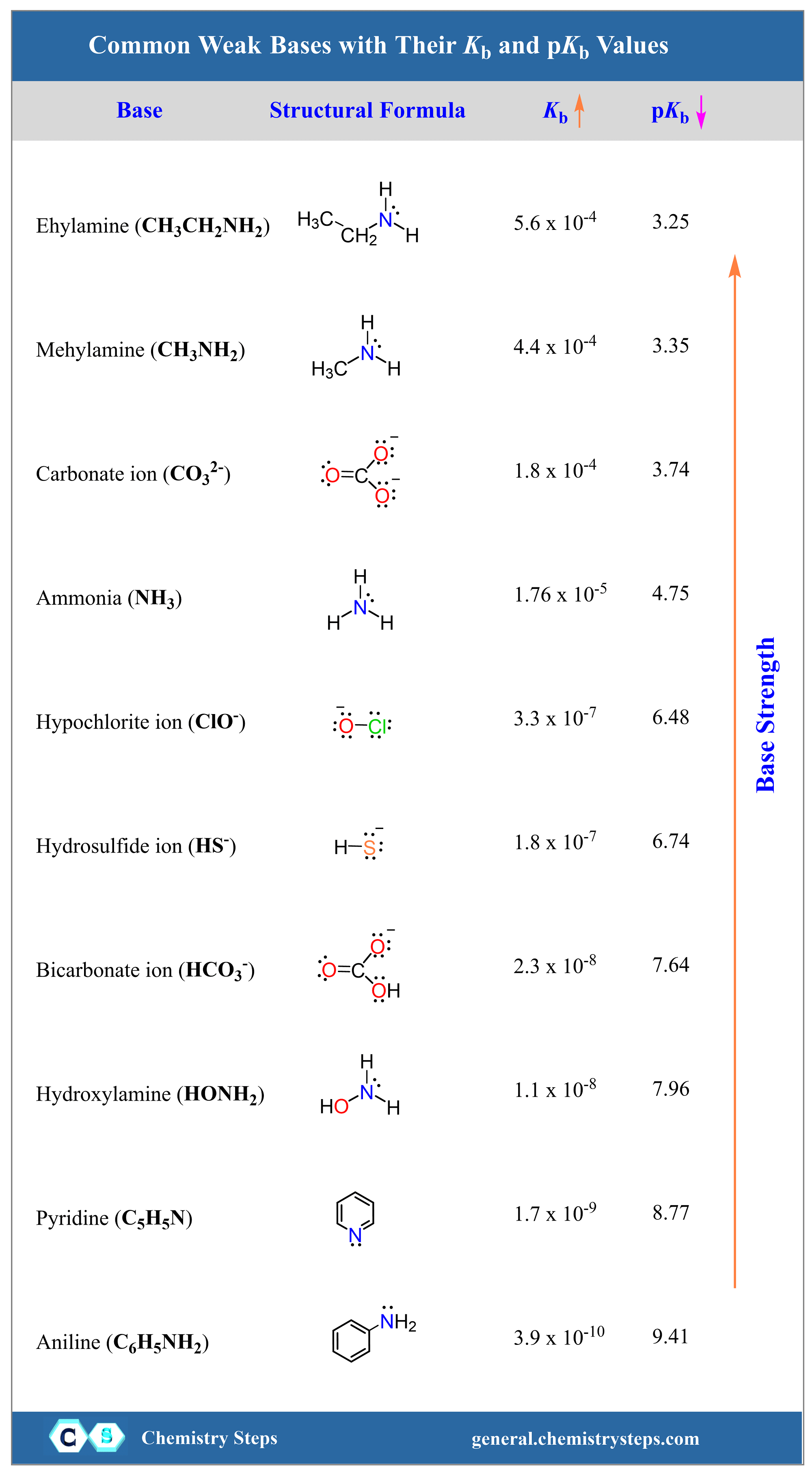
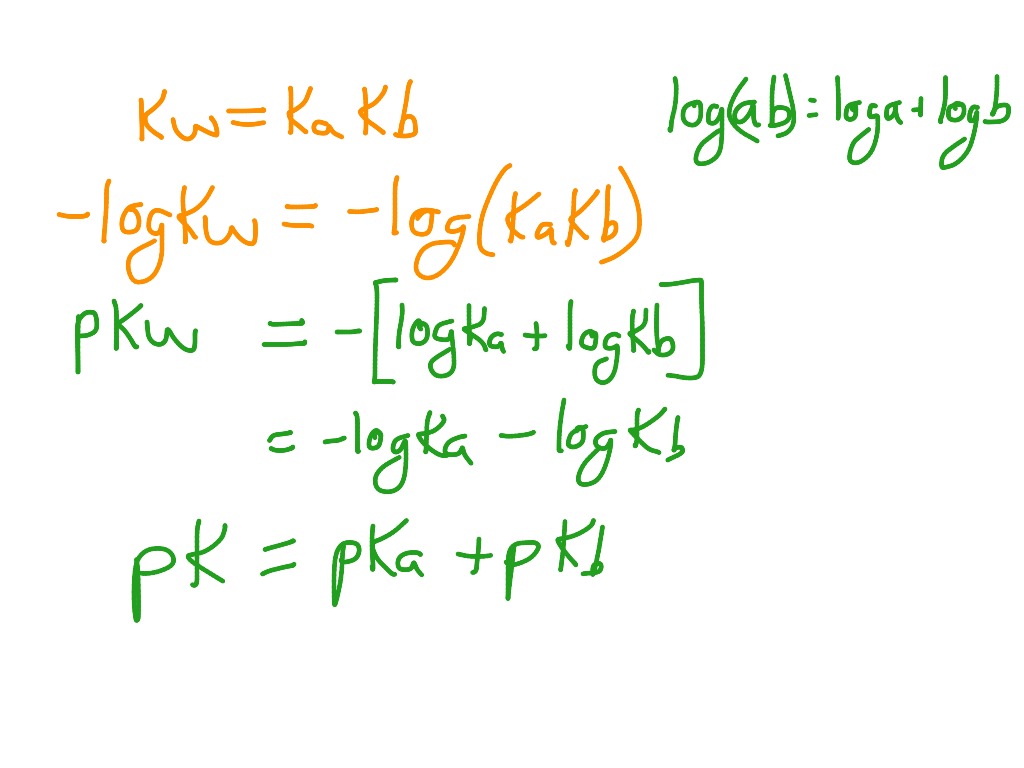pka & pkb definition and formula |How to find the Strength of an acid and base by pka & pkb values - YouTube
![SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10-pH pOH = log10[OH-]; [OH:] = SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10-pH pOH = log10[OH-]; [OH:] =](https://cdn.numerade.com/ask_images/e8201b5be62a4380a6d959f8ef534509.jpg)
SOLVED: Usefulequations: pH pKa + log ([conjV[WA]) where WA = weak acid pOH = pKb + log ([conjV[WB]) where WB weak base pH = -log1o[HgOt]; [H3Ot] = 10-pH pOH = log10[OH-]; [OH:] =
What is the value of pKb (CH3COO-), if λm= 7.8 and λm^∞ = 390 for 0.04M of a CH3COOH solution at 25°C? - Quora








![Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com](https://media.cheggcdn.com/media/14b/14b35f43-4468-42d2-a7e2-d924b3fd6eba/phpQPtBXO.png)