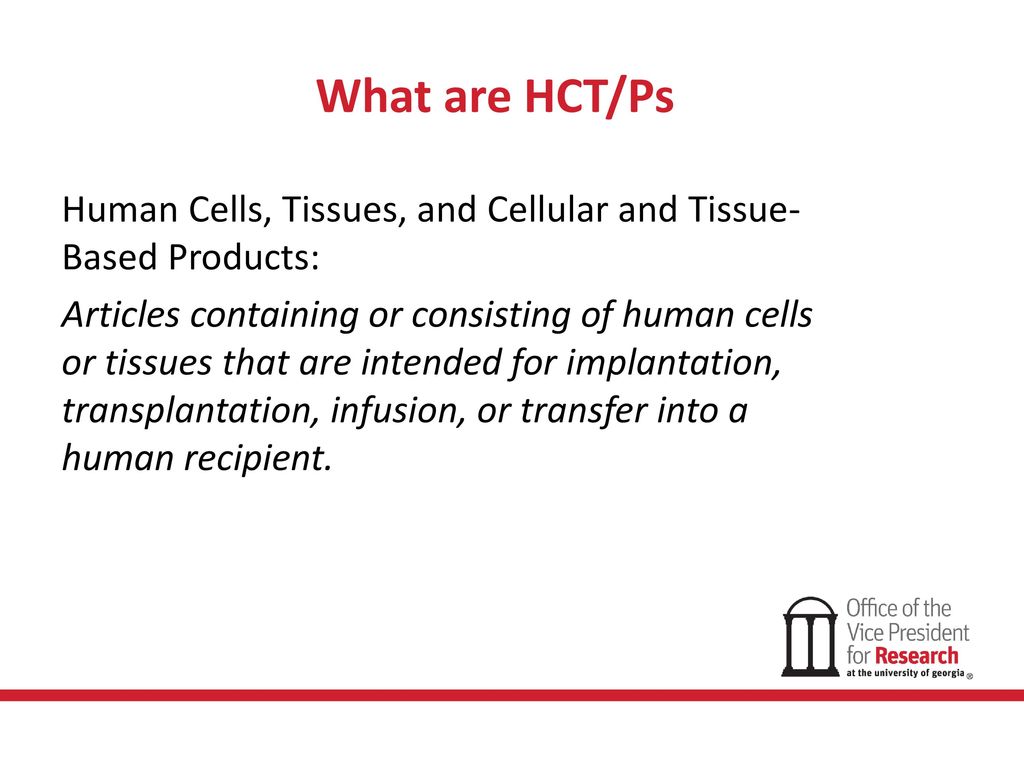
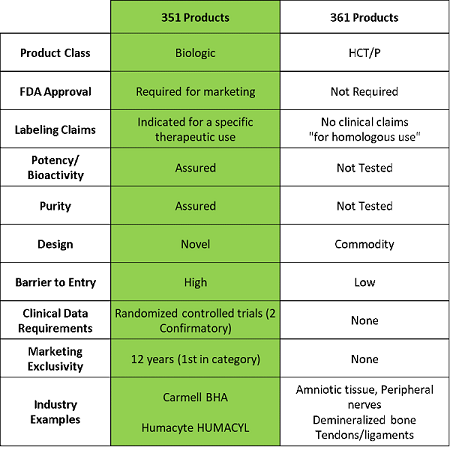
Evaluating the FDA regenerative medicine framework: opportunities for stakeholders | Regenerative Medicine

FDA Issues First Untitled Letter of the Year to HCT/P Manufacturer | Sheppard Mullin Richter & Hampton LLP - JDSupra

FDA Issues Final Guidance Documents on HCT/Ps, Announces a Three Year Period of Enforcement Discretion for Certain HCT/Ps for Autologous Use (Part I of “The FDA's Comprehensive Regenerative Medicine Policy Framework”)
Guidance for Industry: Certain Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) Recovered From Donors

PPT – HCT/P Contamination Prevention and Biologic Product Sterility Regulations Applicable to PBSCs PowerPoint presentation | free to view - id: 22e501-MGU3O