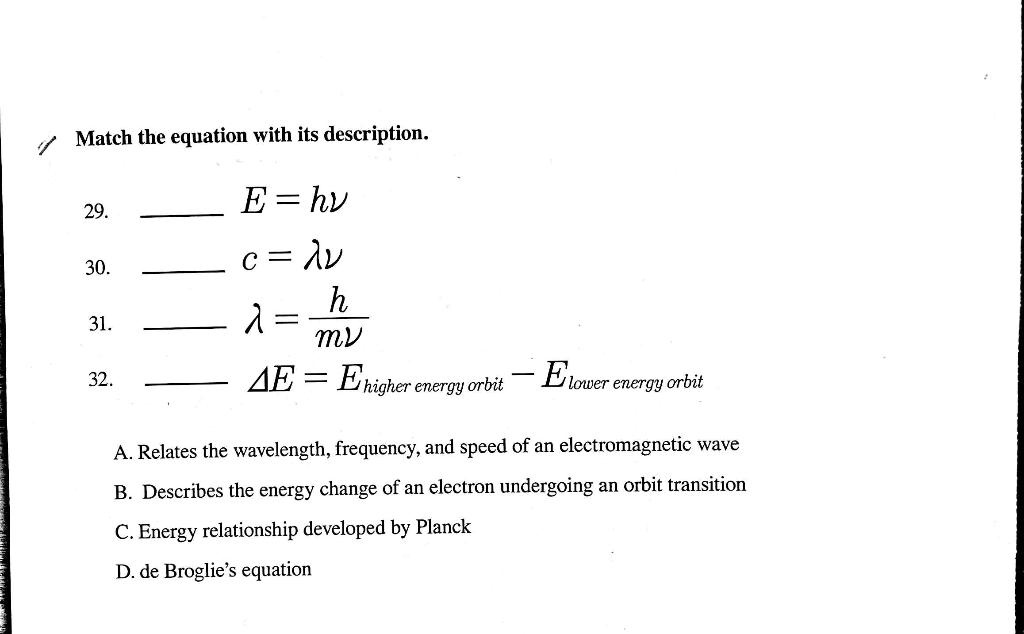
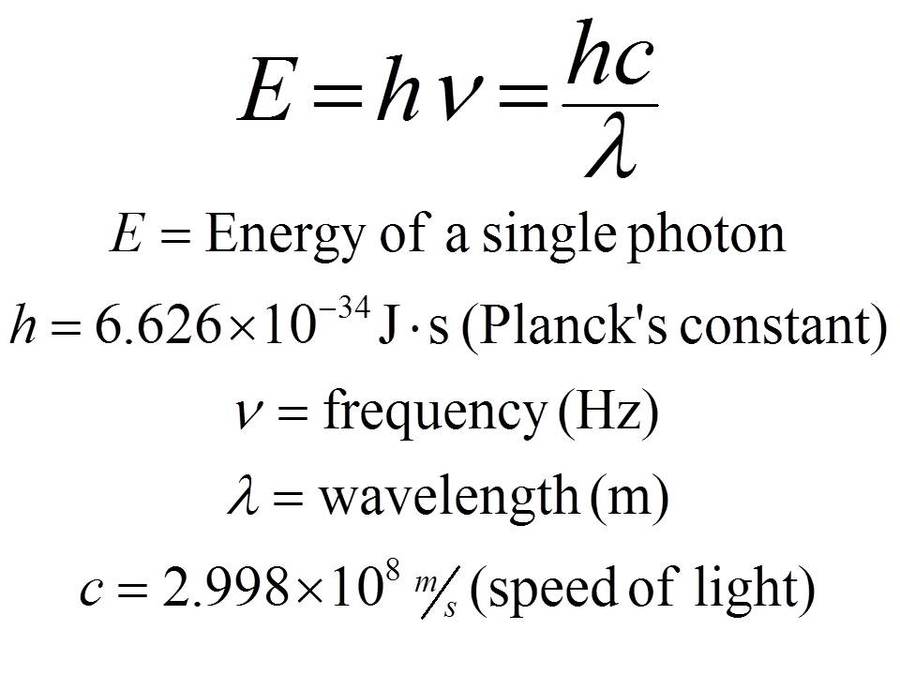
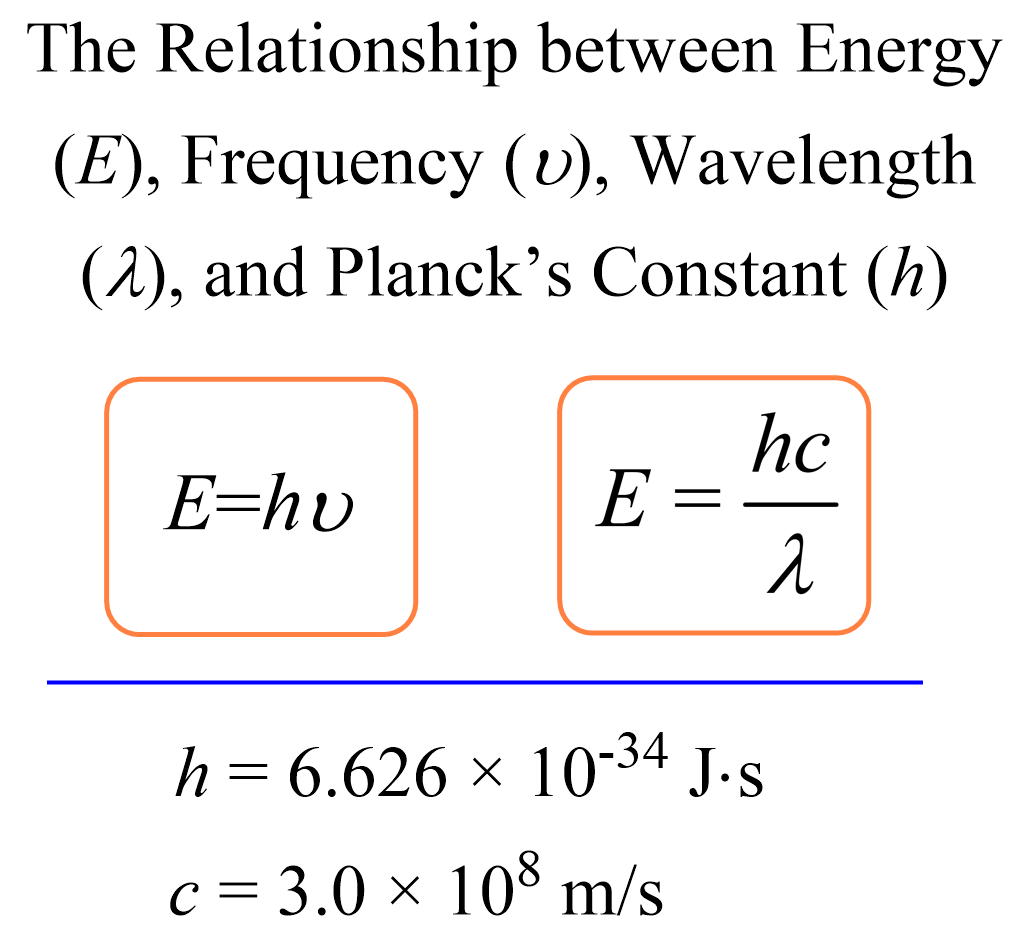
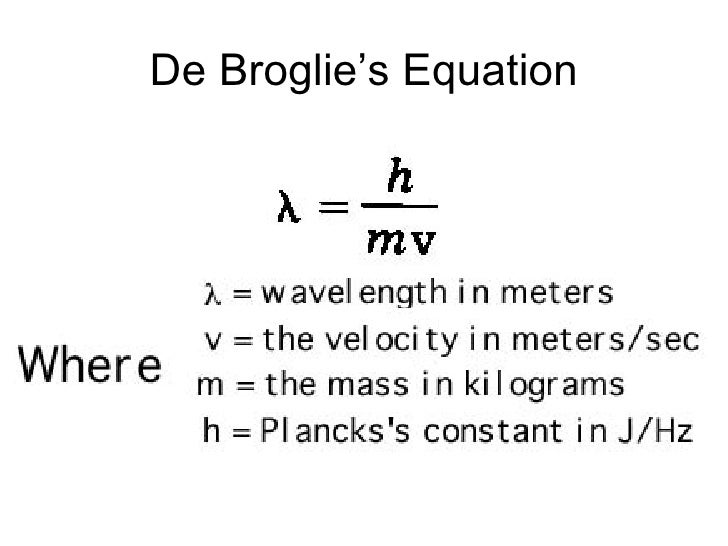
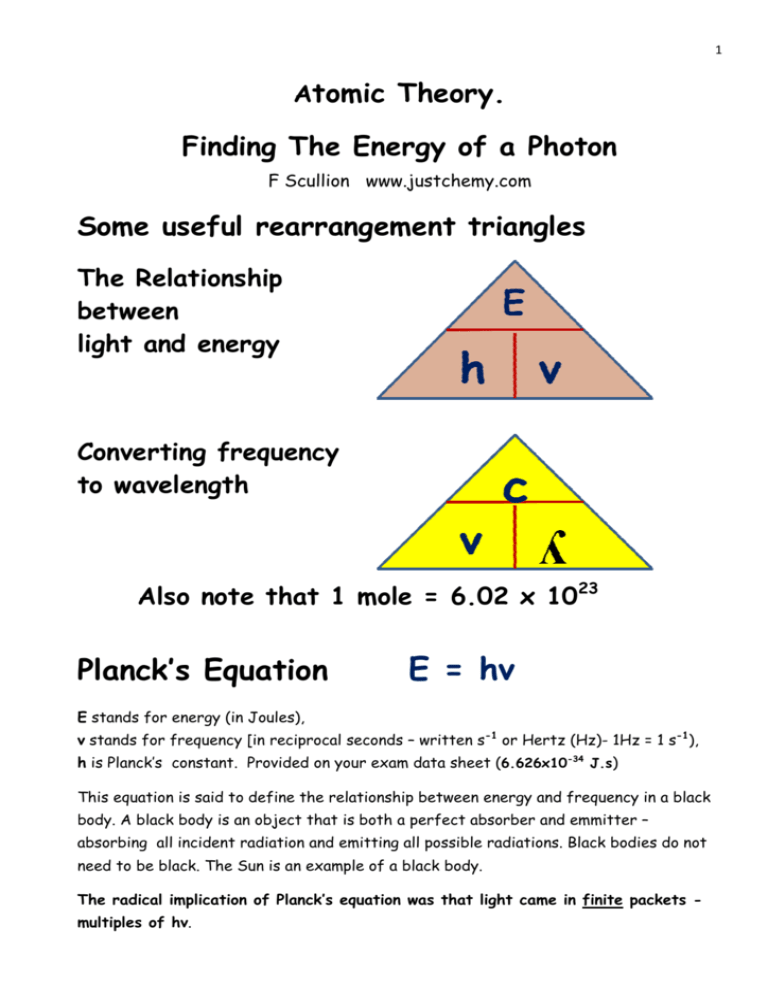
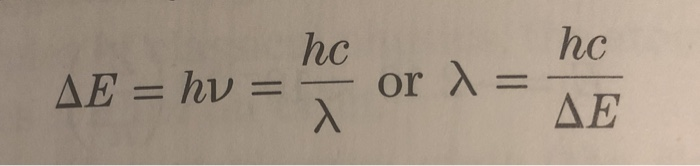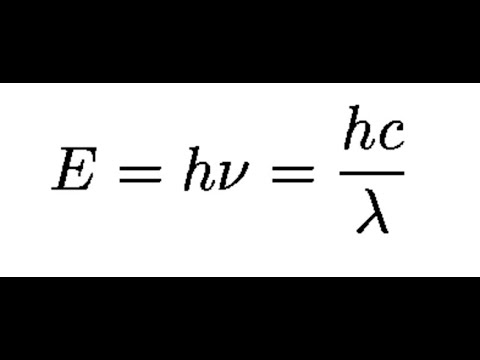The formula for kinetic mass of photon is:where h is Planck's constant and v,lambda, c are frequency, wavelength and speed of photon respectively.
SOLVED: E = hv (v+3)-hv(v+z)x where X is the "anharmonicity constant" for the molecule. In fact; this anharmonicity constant is related to the bond energy De! So we can re-write the energy
The terminology of different parts of the electromagnetic spectrum is given in the text. Use the formula E = hv - Sarthaks eConnect | Largest Online Education Community
Using the two equations E=hv and c=lambda v derive an equation expressing E in terms of h,c and lambda.

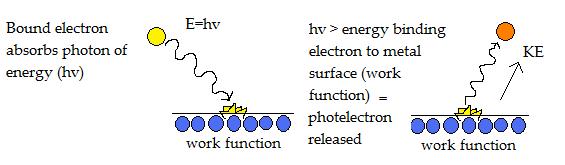
Physicist Page - Einstein's Quantum Theory of Light (Photoelectric Effect) In 1905, the great Albert Einstein, published a paper in which he outlines a possible explanation for the photoelectric effect. According to
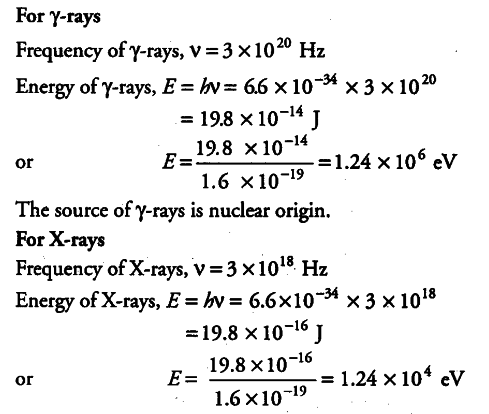
The terminology of different parts of the electromagnetic - CBSE Class 12 Physics - Learn CBSE Forum