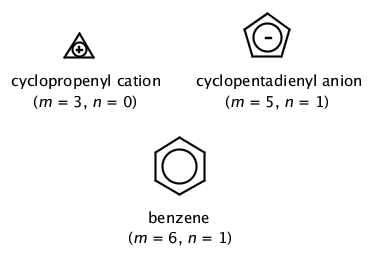
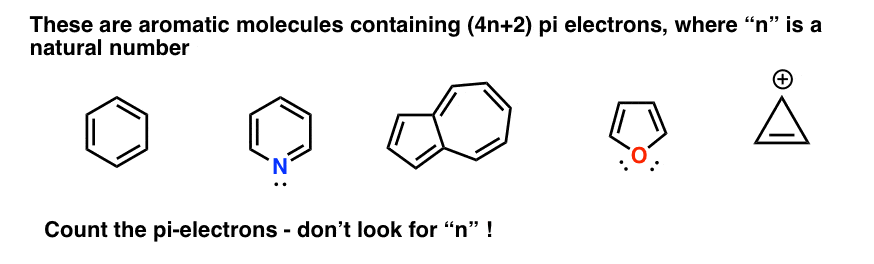
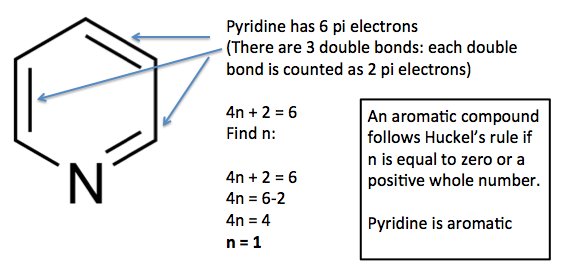
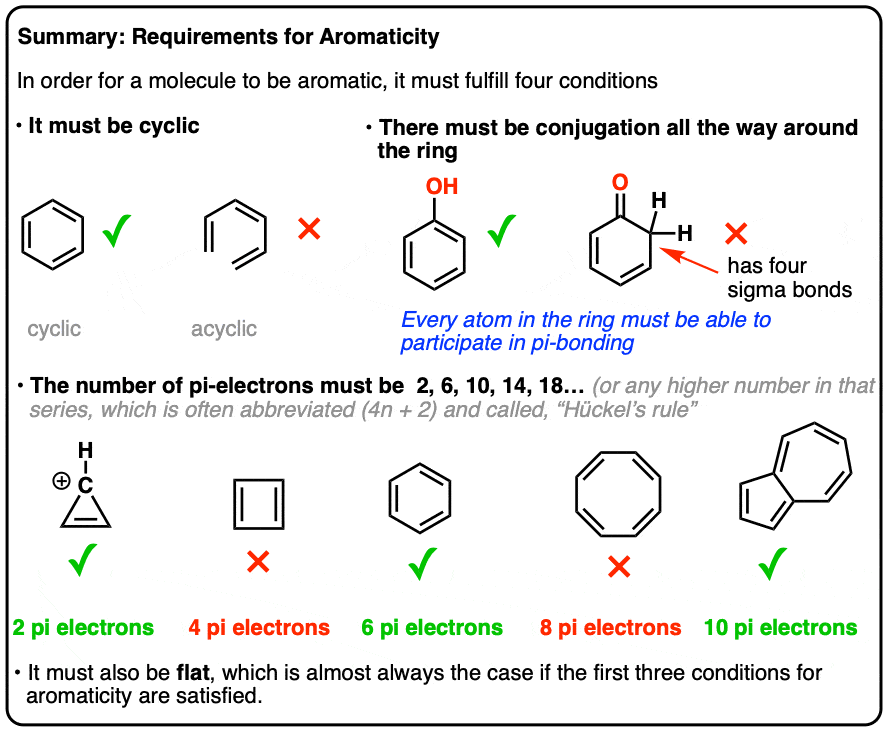
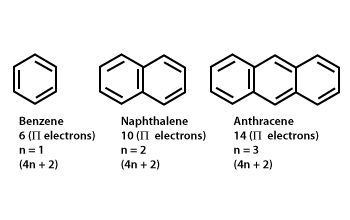
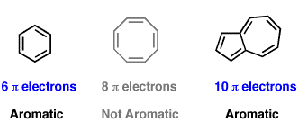
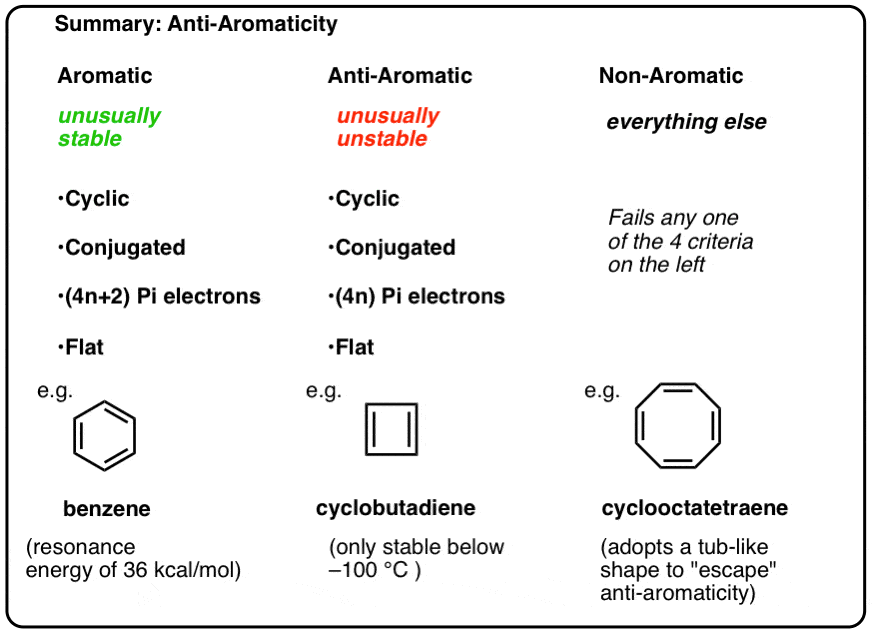
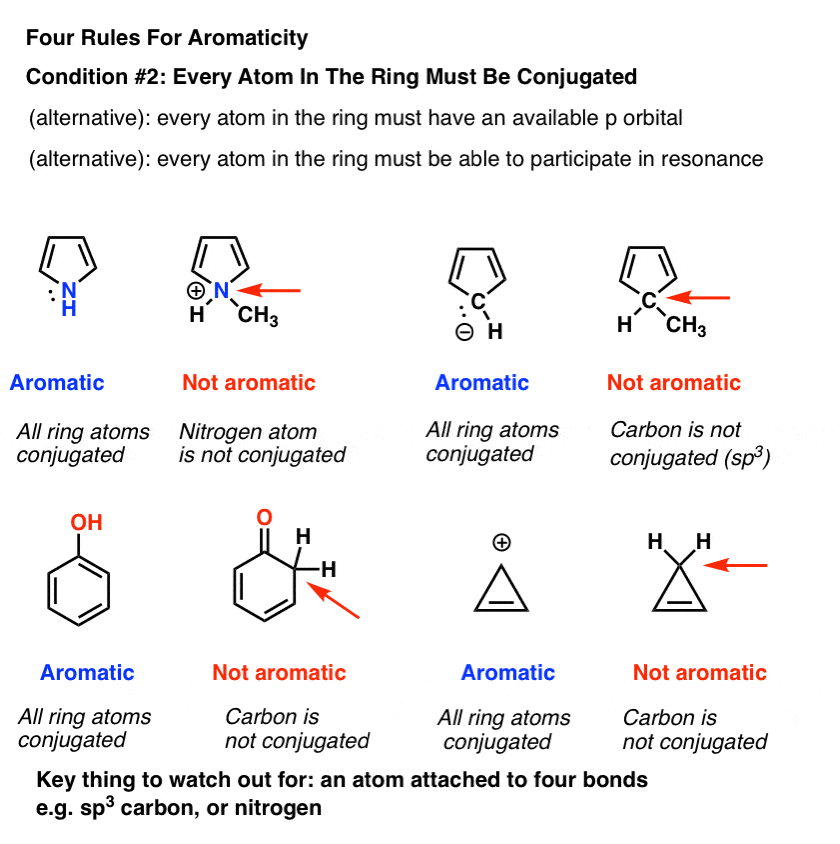
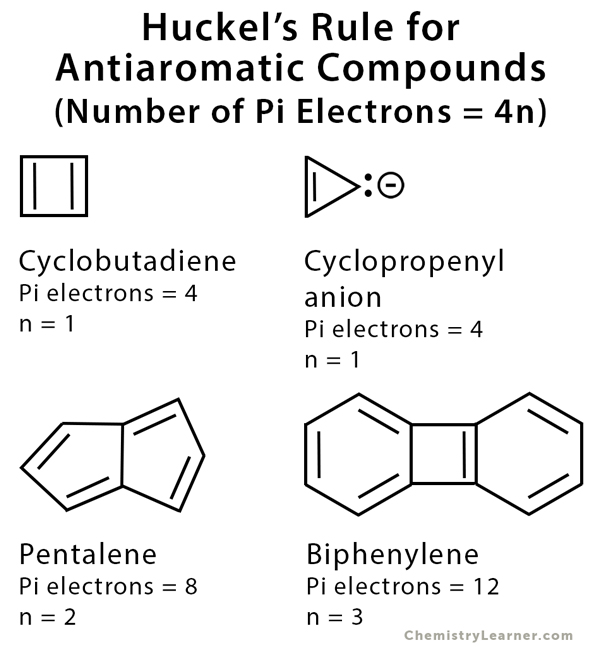
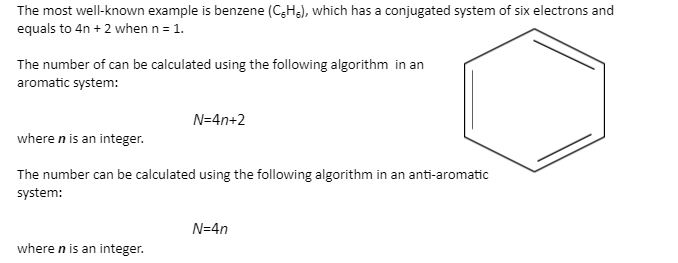
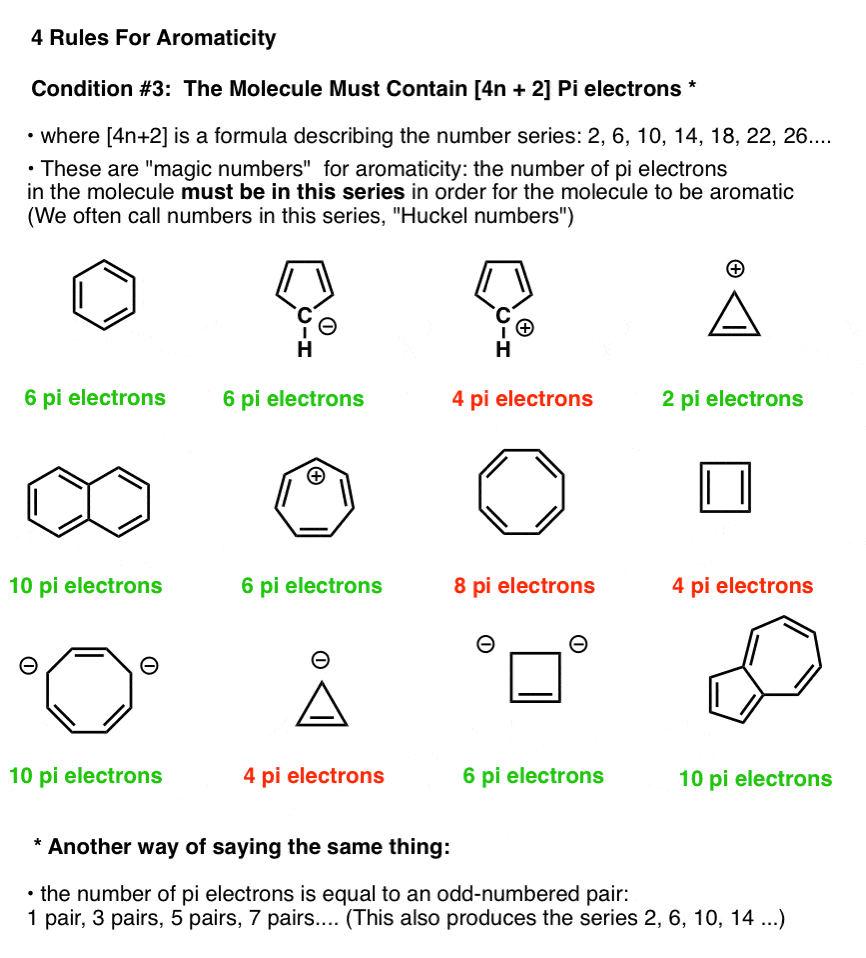
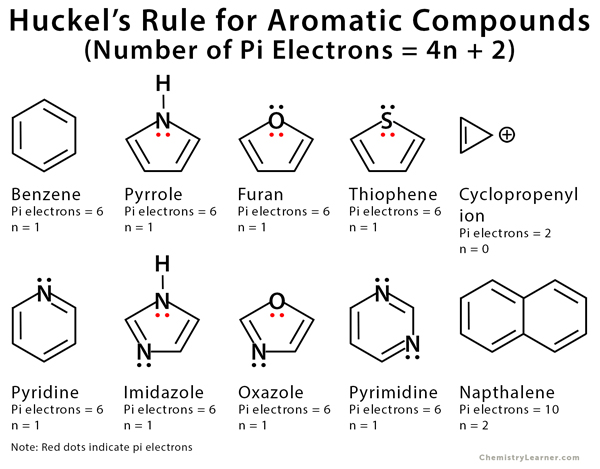
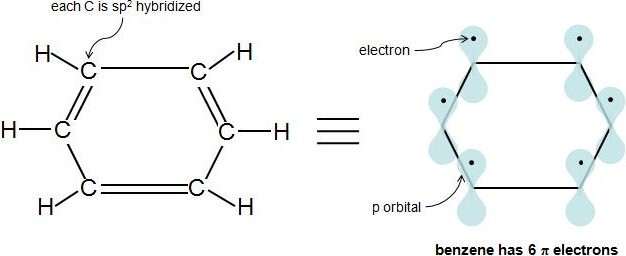
The ring systems having the following characteristics are aromatic:(i) Planar ring containing conjugated pi bonds.(ii) Complete delocalization of the pi electrons in ring system i.e., each atom in the ring has unhybridised

Classics Illustrated: Clar's Sextet and Hückel's (4n + 2) π-Electron Rules | The Journal of Physical Chemistry C

A Disrotatory 4n+2 electron anti-aromatic Möbius transition state for a thermal electrocyclic reaction. | Henry Rzepa's Blog
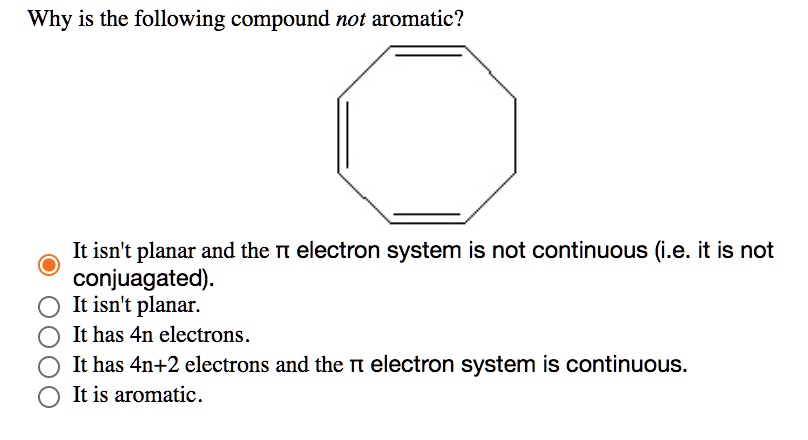
SOLVED: Why is the following compound not aromatic? It isntt planar and the TT electron system is not continuous (i.e: it is not conjuagated): It isn't planar: It has 4n electrons It


.jpg?revision=1)